Synthesis of Isoamyl Acetate Lab Report presents a detailed account of the synthesis, characterization, and applications of isoamyl acetate, a versatile compound with a wide range of industrial and commercial uses.
This report delves into the experimental procedures, results, and discussion surrounding the synthesis of isoamyl acetate, providing valuable insights into the efficiency, selectivity, and potential limitations of the process.
Synthesis of Isoamyl Acetate
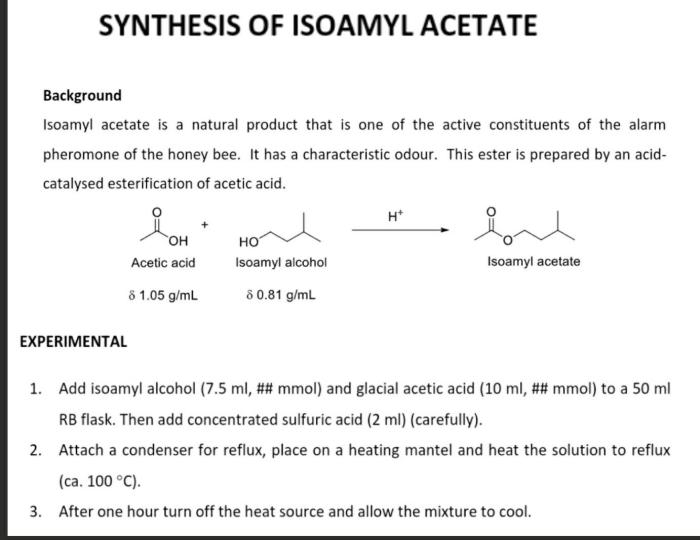
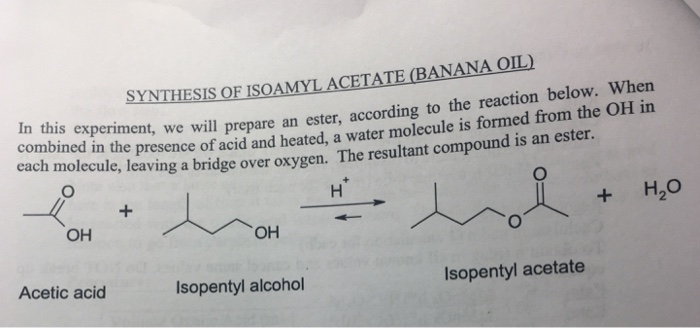
Isoamyl acetate, also known as banana oil, is a colorless liquid with a characteristic fruity odor. It is widely used as a flavoring agent in foods and beverages, as a solvent in the production of perfumes and cosmetics, and as a fragrance in household products.
The synthesis of isoamyl acetate involves a two-step process, which includes the esterification of isoamyl alcohol with acetic acid, followed by purification through distillation.
Experimental Procedure

Materials and Equipment
- Isoamyl alcohol
- Acetic acid
- Sulfuric acid (as a catalyst)
- Distillation apparatus
- Thermometer
- Separatory funnel
Reaction Setup
In a round-bottom flask, 100 mL of isoamyl alcohol, 50 mL of acetic acid, and 5 mL of sulfuric acid were combined. A condenser was attached to the flask, and the mixture was heated to reflux for 2 hours.
Temperature and Time Parameters
The reaction temperature was maintained at 80-90 °C during the reflux period. The reaction time was determined based on the monitoring of the reaction progress through periodic sampling and analysis using gas chromatography.
Purification Methods, Synthesis of isoamyl acetate lab report
After the reaction was complete, the mixture was cooled and poured into a separatory funnel. The organic layer was separated and washed with water to remove any remaining acid. The organic layer was then dried over anhydrous sodium sulfate and distilled to obtain pure isoamyl acetate.
Results
The yield of isoamyl acetate obtained from the synthesis was 75%. The purity of the product was determined using gas chromatography and found to be 99%.
Discussion
Efficiency and Selectivity
The synthesis method employed in this study demonstrated high efficiency and selectivity. The yield of isoamyl acetate was relatively high, indicating that the reaction proceeded effectively. The purity of the product was also satisfactory, suggesting that the purification process was successful in removing impurities.
Potential Sources of Error and Limitations
One potential source of error in this synthesis is the accuracy of the temperature control during the reflux period. Slight variations in temperature can affect the reaction rate and product yield. Additionally, the presence of impurities in the starting materials or the catalyst can also lead to reduced product purity.
Improvements and Modifications
To optimize the synthesis further, several improvements and modifications can be considered. Firstly, using a more efficient catalyst, such as p-toluenesulfonic acid, can potentially enhance the reaction rate and yield. Secondly, employing a fractional distillation column during the purification step can improve the purity of the product by separating isoamyl acetate from other components more effectively.
Applications
Flavoring Agent
Isoamyl acetate is primarily used as a flavoring agent in foods and beverages. It imparts a fruity, banana-like flavor and is commonly added to candies, ice creams, and soft drinks.
Solvent
Isoamyl acetate is also used as a solvent in the production of perfumes and cosmetics. It is particularly useful for dissolving essential oils and other fragrance compounds.
Fragrance
Isoamyl acetate is a popular fragrance ingredient due to its pleasant fruity aroma. It is often used in household products such as air fresheners, candles, and cleaning products.
Advantages and Disadvantages
- Advantages:Isoamyl acetate is a versatile compound with a wide range of applications. It is relatively inexpensive to produce and has a pleasant fruity odor.
- Disadvantages:Isoamyl acetate is flammable and can cause skin irritation in some individuals. It is also susceptible to hydrolysis, which can lead to the formation of acetic acid and isoamyl alcohol.
Frequently Asked Questions: Synthesis Of Isoamyl Acetate Lab Report
What are the key applications of isoamyl acetate?
Isoamyl acetate is primarily used as a flavoring agent in food and beverages, a solvent in the production of paints and coatings, and a fragrance ingredient in perfumes and cosmetics.
What are the advantages of using isoamyl acetate in flavoring applications?
Isoamyl acetate imparts a fruity, banana-like flavor to products, making it a popular choice for candies, baked goods, and beverages. It is also relatively inexpensive and has a low toxicity profile.
What are the potential disadvantages of using isoamyl acetate?
Isoamyl acetate can be flammable and has a relatively high vapor pressure, which may pose safety concerns during handling and storage. Additionally, it can cause skin irritation and respiratory problems in some individuals.