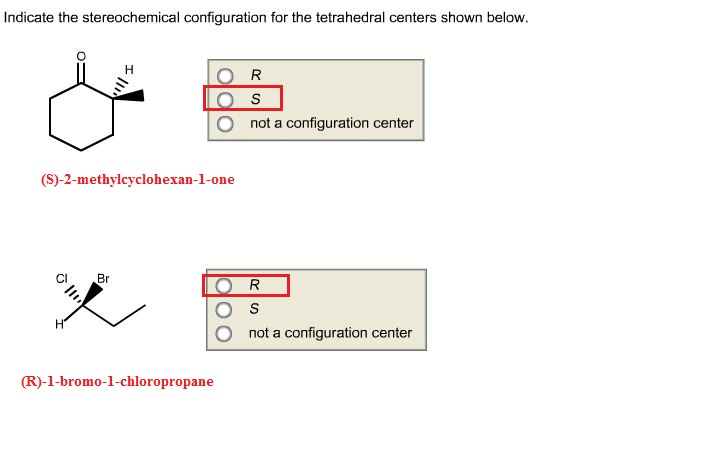
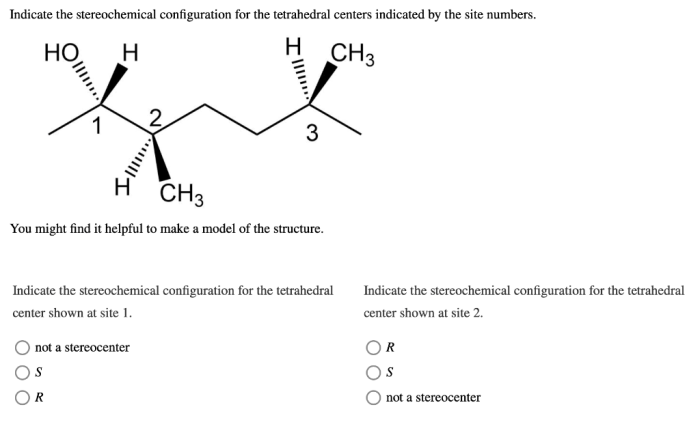
Indicate the stereochemical configuration for the tetrahedral centers. – Delving into the realm of stereochemistry, we encounter the concept of stereochemical configuration, a crucial aspect in understanding the spatial arrangement of atoms within molecules. This guide embarks on a journey to explore the stereochemical configuration of tetrahedral centers, unraveling their significance and providing practical methods for their determination.
Tetrahedral centers, characterized by their four bonds extending towards the corners of a tetrahedron, exhibit distinct stereochemical configurations that dictate the orientation of these bonds in space. Understanding these configurations is paramount in various fields of chemistry, including organic synthesis, drug design, and materials science.
Tetrahedral Centers
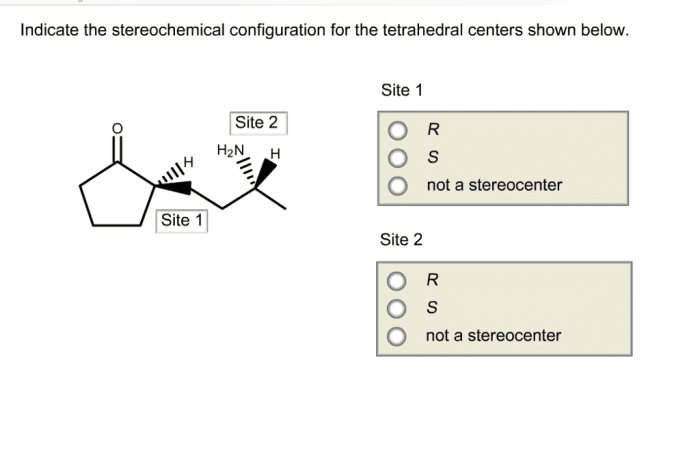
Tetrahedral centers are atoms that are bonded to four other atoms in a tetrahedral geometry. The tetrahedral geometry arises from the hybridization of the central atom’s atomic orbitals, which results in four equivalent sp 3hybrid orbitals.
The bonding in tetrahedral centers is characterized by the formation of four sigma bonds, one from each of the four sp 3hybrid orbitals of the central atom to each of the four bonded atoms.
Stereochemical Configuration
The stereochemical configuration of a tetrahedral center refers to the spatial arrangement of the four atoms bonded to the central atom. The stereochemical configuration is determined by the relative orientations of the four substituents around the central atom.
There are two possible stereochemical configurations for a tetrahedral center: the Rconfiguration and the Sconfiguration. The Rand Sconfigurations are determined by the Cahn-Ingold-Prelog (CIP) priority rules.
To determine the stereochemical configuration of a tetrahedral center, the four substituents are assigned priorities based on their atomic numbers. The substituent with the highest atomic number is assigned the highest priority, and the substituent with the lowest atomic number is assigned the lowest priority.
The remaining two substituents are assigned priorities based on the atomic numbers of the atoms directly bonded to them.
Once the substituents have been assigned priorities, the tetrahedral center is oriented so that the substituent with the lowest priority is pointing away from the viewer. The remaining three substituents are then examined in a clockwise direction. If the order of priority of the substituents is clockwise, the stereochemical configuration is R. If the order of priority of the substituents is counterclockwise, the stereochemical configuration is S.
Examples of Stereochemical Configuration
The following are examples of molecules with tetrahedral centers and different stereochemical configurations:
- Methane (CH 4): The four hydrogen atoms are bonded to the carbon atom in a tetrahedral geometry. The stereochemical configuration of the carbon atom is not specified because all four substituents are identical.
- Chloromethane (CH 3Cl): The chlorine atom is bonded to the carbon atom in a tetrahedral geometry. The stereochemical configuration of the carbon atom is Rbecause the order of priority of the substituents (Cl > H) is clockwise.
- 2-Bromobutane (CH 3CHBrCH 3): The bromine atom is bonded to the second carbon atom in a tetrahedral geometry. The stereochemical configuration of the second carbon atom is Sbecause the order of priority of the substituents (Br > CH 3> H) is counterclockwise.
Methods for Determining Stereochemical Configuration, Indicate the stereochemical configuration for the tetrahedral centers.
There are a number of different methods that can be used to determine the stereochemical configuration of tetrahedral centers. These methods include:
- X-ray crystallography: X-ray crystallography can be used to determine the exact positions of the atoms in a molecule, including the stereochemical configuration of any tetrahedral centers.
- Nuclear magnetic resonance (NMR) spectroscopy: NMR spectroscopy can be used to determine the relative orientations of the substituents around a tetrahedral center. This information can then be used to determine the stereochemical configuration of the center.
- Chiral chromatography: Chiral chromatography can be used to separate enantiomers, which are molecules that have the same molecular formula but different stereochemical configurations. This information can then be used to determine the stereochemical configuration of the tetrahedral center.
Applications of Stereochemical Configuration
The stereochemical configuration of tetrahedral centers has a number of important applications in chemistry. For example, the stereochemical configuration of a molecule can affect its biological activity, its reactivity, and its physical properties.
The stereochemical configuration of drugs is particularly important because it can affect the drug’s ability to interact with its target. For example, the enantiomer of a drug that is active may have no activity or even be harmful.
The stereochemical configuration of molecules can also affect their reactivity. For example, the enantiomers of a molecule may react at different rates with different reagents.
The stereochemical configuration of molecules can also affect their physical properties. For example, the enantiomers of a molecule may have different melting points, boiling points, and solubilities.
Detailed FAQs: Indicate The Stereochemical Configuration For The Tetrahedral Centers.
What is the significance of stereochemical configuration?
Stereochemical configuration determines the spatial arrangement of atoms, influencing molecular properties such as reactivity, selectivity, and biological activity.
How can I determine the stereochemical configuration of a tetrahedral center?
Various methods exist, including NMR spectroscopy, X-ray crystallography, and chiroptical techniques, each with its advantages and limitations.
What are the applications of stereochemical configuration in chemistry?
Stereochemical configuration finds applications in drug design, asymmetric synthesis, and the development of chiral materials with tailored properties.